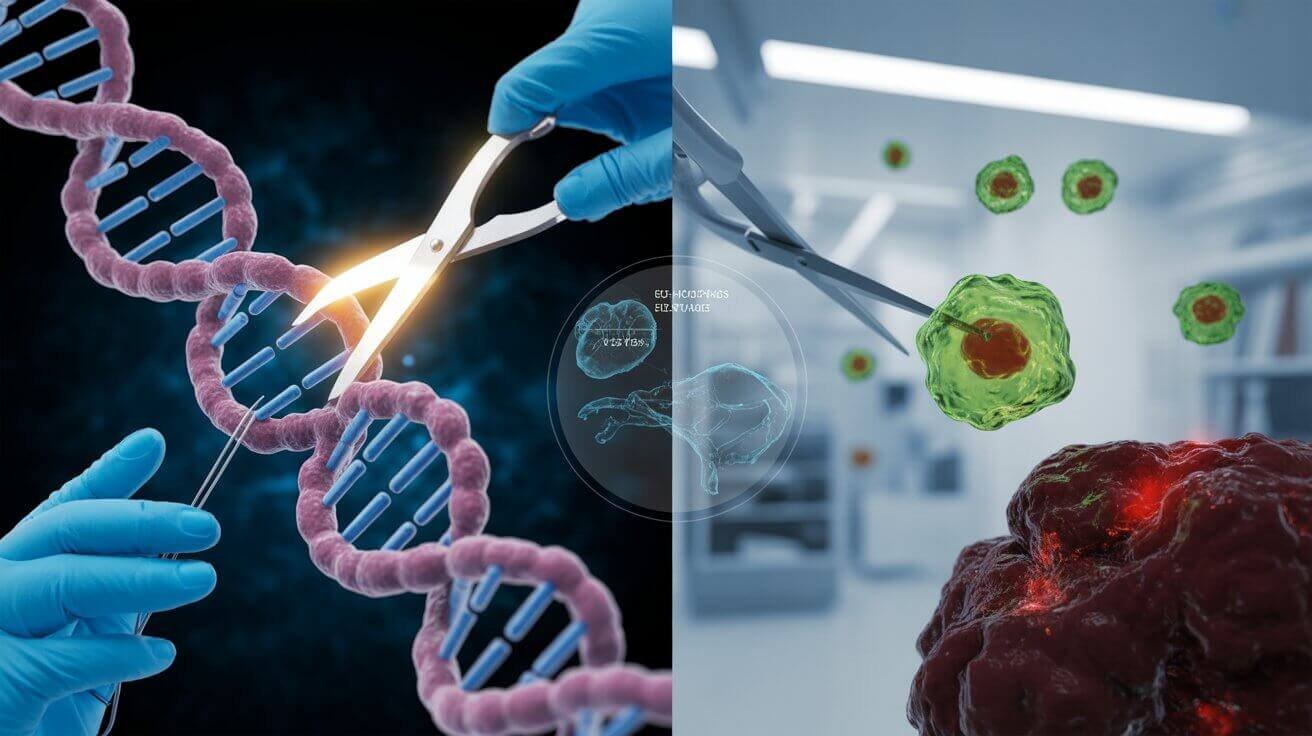
Medicine just reached a turning point. Moreover, personalized cancer therapy is no longer a distant dream—it’s happening right now in hospitals across the world. With the FDA’s recent approval of the first CRISPR-based treatment and breakthrough advances in CAR-T cell therapy, we’re witnessing the birth of truly individualized medicine. Personalized cancer therapy represents a fundamental shift from one-size-fits-all treatments to therapies designed specifically for each patient’s unique genetic makeup.
So what makes this moment so significant? Simply put, we’re moving beyond traditional chemotherapy’s broad-brush approach to precision strikes against cancer cells. This transformation stems from our growing understanding that every patient’s cancer is as unique as their fingerprint, requiring equally unique solutions.
The Science Behind Personalized Cancer Therapy
Traditional cancer treatments have always faced a fundamental challenge: they attack both healthy and cancerous cells. However, personalized cancer therapy changes this equation entirely. Instead of using generalized treatments, doctors can now analyze a patient’s specific genetic mutations and design targeted interventions.
CRISPR technology serves as the foundation for many of these advances. The CRISPR system is a precise genome editing tool that has the potential to alter specific genetic mutations that drive tumor growth and spread. Unlike traditional treatments, CRISPR can target the exact DNA sequences responsible for a patient’s cancer.
Furthermore, these personalized approaches work by identifying actionable genes—specific genetic changes that can be targeted with existing or experimental drugs. When doctors find these mutations, they can select treatments most likely to work for that individual patient. This represents a dramatic departure from the trial-and-error approach that has dominated cancer care for decades.
Recent advances have made personalized cancer therapy more accessible than ever before. Cancer is a severe disease that substantially jeopardizes global health, but genome editing offers an ideal way to implement personalized treatments by allowing direct modification of pro-tumor genes.
CRISPR: The Gene Editing Breakthrough Transforming Treatment
CRISPR technology has moved from laboratory curiosity to life-saving medicine in record time. Consequently, the FDA approved the world’s first CRISPR-based medicine, Casgevy, in December 2023 for treating sickle cell disease. While this approval focused on a blood disorder, it opens the door for CRISPR applications in cancer treatment.
The power of CRISPR lies in its precision. Think of it as molecular scissors that can cut DNA at exactly the right spot, then either remove problematic sequences or insert beneficial ones. In cancer applications, researchers use CRISPR to enhance immune cells, making them better at recognizing and destroying tumors.
For example, scientists are using CRISPR to create “super-charged” T cells for immunotherapy. CRISPR is used to remove three genes: two that can interfere with cancer recognition and another that limits the cells’ cancer-killing abilities. This genetic modification helps T cells persist longer in the body and fight cancer more effectively.
Additionally, CRISPR applications extend beyond direct treatment. Researchers use the technology to create better cancer models for drug testing and to identify new therapeutic targets. This accelerates the development of personalized cancer therapy options for patients who previously had limited choices.
CAR-T Cell Therapy: Engineering Immune Cells to Fight Cancer
CAR-T cell therapy represents one of the most successful examples of personalized cancer therapy currently available. The treatment involves extracting a patient’s T cells, genetically modifying them to better recognize cancer, then reinfusing them to fight the disease.
The results speak for themselves. In one clinical trial involving people with advanced follicular lymphoma, the CAR-T cell therapy eliminated cancer in nearly 80% of patients, with many remaining disease-free three years later. For large cell lymphoma, more than 30% of participants were alive without evidence of cancer five years after treatment.
However, CAR-T therapy works best for blood cancers. Solid tumors present additional challenges because they create immunosuppressive environments that can neutralize CAR-T cells. Nevertheless, researchers are developing innovative solutions to overcome these obstacles.
Recent advances include multi-specific CAR-T cells that recognize more than one antigen, helping overcome tumor heterogeneity. Scientists are also creating “Boolean logic gated CAR-T cells” with improved specificity and better safety profiles.
Moreover, the field is expanding beyond cancer. CAR-T cell therapy is now being tested for autoimmune diseases like lupus and type 1 diabetes, demonstrating the versatility of this personalized approach.
Real-World Success Stories and Clinical Breakthroughs
Personalized cancer therapy isn’t just theoretical—it’s saving lives today. Take the example of patients receiving CRISPR-edited T cells at the University of Pennsylvania. While the initial trial involved only three patients, the results were encouraging: the CRISPR-edited cells persisted at least 9 months, versus about 2 months in comparable CAR-T cell therapy studies.
Another breakthrough comes from the development of “off-the-shelf” CAR-T cells. Traditional CAR-T therapy requires extracting cells from each patient individually, which is expensive and time-consuming. However, researchers are developing allogeneic CAR-T cells from healthy donors that could be used for multiple patients.
The economic impact is substantial. FDA approved eight novel cell and gene therapies in 2024, with projections of 10 to 20 approvals annually by 2025. This acceleration reflects growing confidence in personalized cancer therapy approaches.
Furthermore, companies are investing heavily in manufacturing capabilities. The personalized nature of these treatments requires sophisticated production facilities, but automation and standardization are making them more accessible. As production scales up, costs should decrease, making personalized cancer therapy available to more patients.
Overcoming Challenges in Personalized Treatment
Despite impressive progress, personalized cancer therapy faces significant hurdles. The biggest challenge is tumor heterogeneity—even within a single patient, cancer cells can vary dramatically. This diversity means that targeting one genetic mutation might not eliminate all cancer cells.
Cost represents another major barrier. Current CAR-T therapies can cost hundreds of thousands of dollars per patient. However, ongoing efforts to create universal CAR-T cells that work for multiple patients could dramatically reduce these expenses.
Manufacturing complexity also poses challenges. Each personalized treatment requires specialized facilities and expertise. Nevertheless, companies are developing automated systems and standardized protocols to streamline production. The goal is making personalized cancer therapy as routine as traditional chemotherapy.
Safety concerns require ongoing attention. While CRISPR appears safe in early trials, patients need lifelong monitoring for potential long-term effects. The FDA requires companies to follow patients for 15 years to document any adverse events.
Additionally, delivery methods need improvement. Getting gene editing tools into cancer cells efficiently and safely remains challenging. However, researchers are developing better delivery systems, including lipid nanoparticles and novel viral vectors that could improve treatment effectiveness.
The Future of Precision Medicine
Looking ahead, personalized cancer therapy will become increasingly sophisticated. Artificial intelligence is already helping doctors identify which patients will benefit most from specific treatments. Machine learning algorithms can analyze vast amounts of genomic data to predict treatment responses with remarkable accuracy.
Combination therapies represent another frontier. Instead of using single treatments, doctors are combining CRISPR, CAR-T cells, and traditional therapies for maximum effect. Early results suggest these approaches could overcome many current limitations of individual treatments.
The integration of liquid biopsies—blood tests that detect circulating tumor DNA—will enable real-time monitoring of treatment effectiveness. This technology allows doctors to adjust personalized cancer therapy strategies based on how tumors respond, rather than waiting for traditional imaging studies.
Moreover, gene editing technology can be leveraged to develop personalized cancer vaccines and improve immunotherapy response. These vaccines would be unique to each patient’s tumor, training their immune system to recognize and attack cancer cells more effectively.
Prevention will also benefit from these advances. By identifying genetic predispositions to cancer, doctors can recommend personalized screening schedules and preventive interventions before tumors develop. This proactive approach could prevent many cancers entirely.
Practical Steps for Patients Today
If you’re facing a cancer diagnosis, here’s how to access personalized treatment options:
Get Comprehensive Genetic Testing: Ask your oncologist about tumor sequencing to identify actionable mutations. Many insurance plans now cover these tests, and the results can reveal treatment options you might not otherwise consider.
Seek Second Opinions at Academic Centers: Major cancer centers often have access to the latest personalized therapies through clinical trials. Don’t hesitate to travel if necessary—your life may depend on it.
Consider Clinical Trials: Many breakthrough personalized cancer therapy options are only available through research studies. Websites like ClinicalTrials.gov can help you find relevant trials, and patient advocacy organizations often provide assistance navigating the process.
Work with Genetic Counselors: These specialists can help interpret genetic test results and explain how they might affect your treatment options and family members.
Stay Informed: The field moves quickly, and new options become available regularly. Join patient advocacy groups and follow reputable medical news sources to stay current on developments that might benefit you.
A New Era of Hope
Personalized cancer therapy represents more than just medical progress—it embodies hope for millions facing cancer diagnoses. We’re witnessing the emergence of treatments that were pure science fiction just a decade ago. The combination of CRISPR gene editing and enhanced immunotherapies is creating opportunities to not just treat cancer, but potentially cure it.
The path forward isn’t without challenges. Cost, complexity, and the need for specialized expertise will require continued innovation and investment. However, the rapid pace of progress suggests these obstacles are temporary rather than permanent barriers.
As we move through 2025, personalized cancer therapy will become increasingly mainstream. The treatments pioneered in major academic centers today will become standard care at community hospitals tomorrow. This democratization of precision medicine means that geography and resources won’t determine access to life-saving treatments.
For patients, families, and healthcare providers, this represents a fundamental shift in how we think about cancer. Rather than a one-size-fits-all approach, we’re entering an era where every treatment plan is as unique as the person receiving it. That’s not just medical progress—it’s a transformation in what it means to fight cancer.